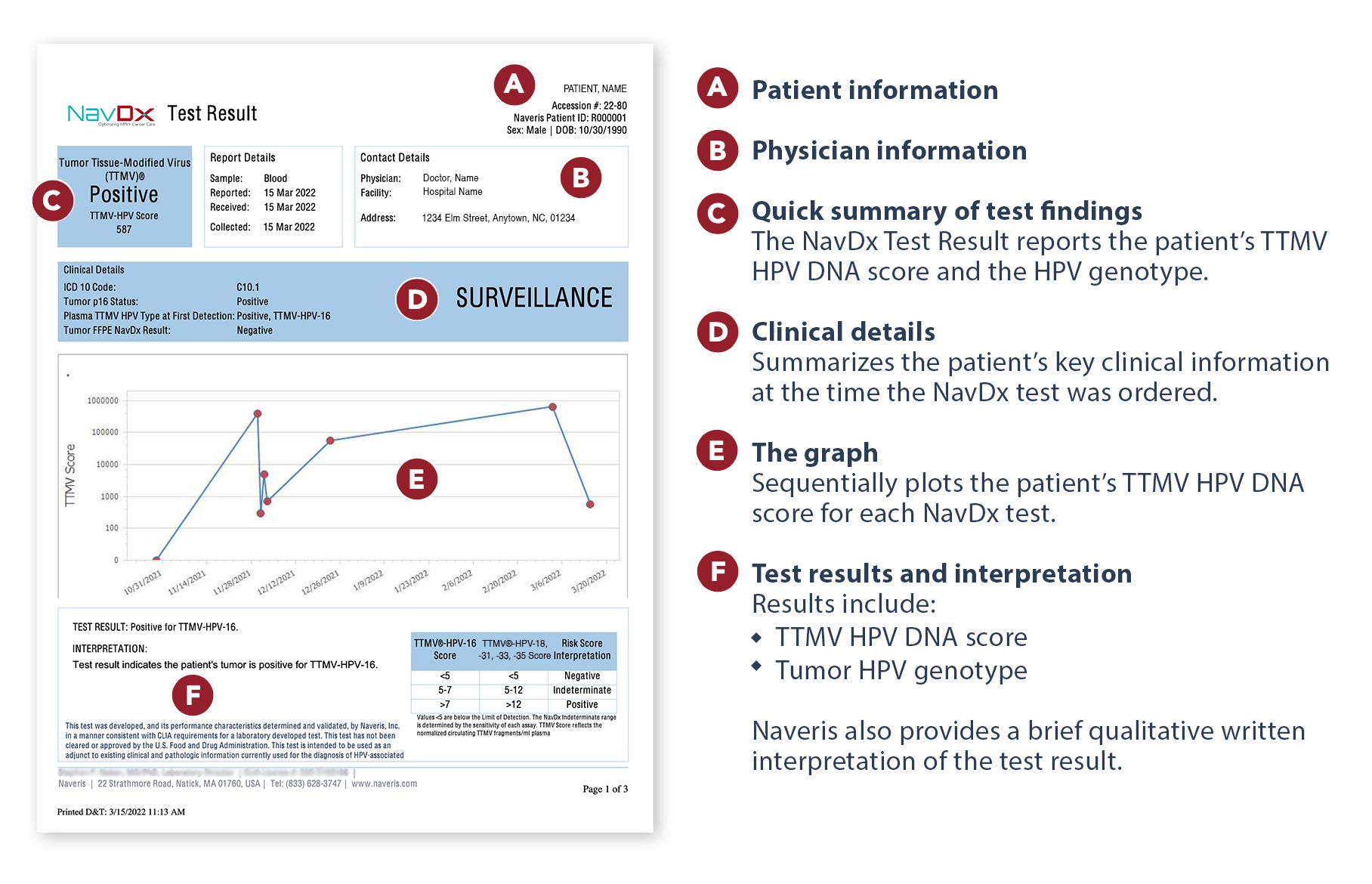
NavDx® is the first and only clinically validated circulating tumor-tissue-modified HPV (TTMV) DNA blood test that can aid in the detection of HPV-driven cancer.¹˒³
NavDx uncovers the presence of HPV+ cancer across the patient care continuum, so treating physicians can intervene earlier, which may result in improved outcomes.¹˒²˒³˒⁴ Use NavDx to optimize clinical management across the care continuum by: